Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Murakami, Tsuyoshi*; Hayashi, Hirokazu
Journal of Nuclear Materials, 558, p.153330_1 - 153330_7, 2022/01
Times Cited Count:0 Percentile:0.01(Materials Science, Multidisciplinary)Excess amounts of dissolution agents, CdCl and ZrCl
and ZrCl , are required to dissolve transuranium (TRU: Pu and minor actinides) nitrides into LiCl-KCl melts at the chemical dissolution step, which is the first step in the reprocessing of used nitride fuels. We propose an electrochemical process where the remaining Zr and Cd are recovered from the melts to be recycled as dissolution agents for the chemical dissolution step, leaving TRU in the melts. Since the initial concentration ratio of CdCl
, are required to dissolve transuranium (TRU: Pu and minor actinides) nitrides into LiCl-KCl melts at the chemical dissolution step, which is the first step in the reprocessing of used nitride fuels. We propose an electrochemical process where the remaining Zr and Cd are recovered from the melts to be recycled as dissolution agents for the chemical dissolution step, leaving TRU in the melts. Since the initial concentration ratio of CdCl /ZrCl
/ZrCl remaining in the melts would depend on the condition of the chemical dissolution step and would vary during the proposed electrochemical recovery process, electrochemical behaviors of Zr and Cd were investigated in LiCl-KCl melts with various concentration ratios of CdCl
remaining in the melts would depend on the condition of the chemical dissolution step and would vary during the proposed electrochemical recovery process, electrochemical behaviors of Zr and Cd were investigated in LiCl-KCl melts with various concentration ratios of CdCl /ZrCl
/ZrCl at 723 K to confirm the basic feasibility of the proposed process. Potentiostatic electrolysis was performed using a liquid Cd cathode at -1.05 V (vs. Ag/AgCl), which was a more positive potential than the redox potentials of TRU on the liquid Cd electrode. The obtained results showed that the current efficiency for recovering Zr and Cd from the melts was as high as 100% regardless of the CdCl
at 723 K to confirm the basic feasibility of the proposed process. Potentiostatic electrolysis was performed using a liquid Cd cathode at -1.05 V (vs. Ag/AgCl), which was a more positive potential than the redox potentials of TRU on the liquid Cd electrode. The obtained results showed that the current efficiency for recovering Zr and Cd from the melts was as high as 100% regardless of the CdCl /ZrCl
/ZrCl concentration ratio in the melts.
concentration ratio in the melts.
Ichihara, Akira; Matsuoka, Leo*; Segawa, Etsuo*; Yokoyama, Keiichi
Physical Review A, 91(4), p.043404_1 - 043404_7, 2015/04
Times Cited Count:13 Percentile:58.58(Optics)We propose a new method for isotope-selective dissociation of diatomic molecules in the gas phase by using two kinds of terahertz-pulse fields. The first field consists of a train of pulses, which composes a frequency comb, excites the selected isotope into highly-rotationally excited state. The second intense pulse field dissociates the excited molecule by further rotational excitations. We performed wave-packet computations using the lithium chlorides 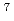 Li
Li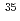 CL and
CL and 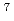 Li
Li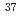 Cl to demonstrate the applicability of our method. Nearly 20% of
Cl to demonstrate the applicability of our method. Nearly 20% of 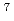 Li
Li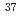 Cl in the lowest rovibrational state is dissociated in the designed pulse fields, while the dissociation probability is negligible in
Cl in the lowest rovibrational state is dissociated in the designed pulse fields, while the dissociation probability is negligible in 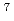 Li
Li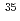 Cl. This method is expected to be applicable to other diatomic molecules, and to molecular ensembles whose rotational states spread in energy.
Cl. This method is expected to be applicable to other diatomic molecules, and to molecular ensembles whose rotational states spread in energy.
Kato, Tetsuya*; Inoue, Tadashi*; Iwai, Takashi; Arai, Yasuo
Journal of Nuclear Materials, 357(1-3), p.105 - 114, 2006/10
Times Cited Count:104 Percentile:98.82(Materials Science, Multidisciplinary)Electrorefining in the molten LiCl-KCl eutectic salt containing actinides(An) and rare-earths(RE) was conducted to recover up to 10 wt% An into liquid Cd cathode, which is much higher than the solubility of An in liquid Cd at the experimental temperature. In the saturated Cd cathode, An and RE were recovered to form a compound of the PuCd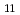 lattice, MCd
lattice, MCd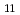 . The separation factors of RE against Pu were defined as (RE/Pu in the Cd cathode)/(RE/Pu in the salt) and calculated for the saturated Cd alloy including MCd
. The separation factors of RE against Pu were defined as (RE/Pu in the Cd cathode)/(RE/Pu in the salt) and calculated for the saturated Cd alloy including MCd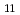 . The separation factors for the Cd cathodes were a little larger than the equilibrium values.
. The separation factors for the Cd cathodes were a little larger than the equilibrium values.
Hayashi, Hirokazu; Minato, Kazuo
Journal of Physics and Chemistry of Solids, 66(2-4), p.422 - 426, 2005/02
Times Cited Count:34 Percentile:75.68(Chemistry, Multidisciplinary)Stability of lanthanide sesquioxides (Ln O
O ; Ln=La, Nd, Gd) in LiCl-KCl eutectic melt is studied. Some lanthanide oxides have been pointed out to form oxide chlorides (LnOCl) in the chloride melts, according to the reaction, Ln
; Ln=La, Nd, Gd) in LiCl-KCl eutectic melt is studied. Some lanthanide oxides have been pointed out to form oxide chlorides (LnOCl) in the chloride melts, according to the reaction, Ln O
O + 2Cl
+ 2Cl = 2LnOCl +O
= 2LnOCl +O . Equilibrium of the reaction for Ln = La, Nd, Gd in LiCl-KCl eutectic melt were studied. Entire La
. Equilibrium of the reaction for Ln = La, Nd, Gd in LiCl-KCl eutectic melt were studied. Entire La O
O and a part of Nd
and a part of Nd O
O converted to LaOCl and NdOCl respectively, though Gd
converted to LaOCl and NdOCl respectively, though Gd O
O remained with a trace amount of GdOCl for 2wt % of Ln
remained with a trace amount of GdOCl for 2wt % of Ln O
O in LiCl-KCl eutectic melt. The equilibrium depends on the free energies of formation of the solid compounds, Ln
in LiCl-KCl eutectic melt. The equilibrium depends on the free energies of formation of the solid compounds, Ln O
O and LnOCl, and that of oxide ion (O
and LnOCl, and that of oxide ion (O ) in the melt. We derive the chemical potential of the oxide ion from the equilibrium using the reported thermochemical data of Ln
) in the melt. We derive the chemical potential of the oxide ion from the equilibrium using the reported thermochemical data of Ln O
O and LnOCl.
and LnOCl.
 /Np couple at liquid Cd and Bi electrodes in LiCl-KCl eutectic melts
/Np couple at liquid Cd and Bi electrodes in LiCl-KCl eutectic meltsShirai, Osamu; Uozumi, Koichi*; Iwai, Takashi; Arai, Yasuo
Journal of Applied Electrochemistry, 34(3), p.323 - 330, 2004/03
Times Cited Count:28 Percentile:52.41(Electrochemistry)The electrode reactions of the Np /Np couple at liquid Cd and Bi electrodes were investigated by cyclic voltammetry at 723, 773 and 823 K in LiCl-KCl eutectic melt. It was found that the diffusion of Np
/Np couple at liquid Cd and Bi electrodes were investigated by cyclic voltammetry at 723, 773 and 823 K in LiCl-KCl eutectic melt. It was found that the diffusion of Np in the salt phase was a rate-determining step in the cathodic reaction when the concentration of NpCl
in the salt phase was a rate-determining step in the cathodic reaction when the concentration of NpCl was less than about 1 wt.% and the liquid Cd or Bi phase was not saturated with Np. The redox potentials of the Np
was less than about 1 wt.% and the liquid Cd or Bi phase was not saturated with Np. The redox potentials of the Np /Np couple at liquid Cd electrode at 723, 773 and 823 K were observed more positively than those at Mo electrode by 0.158, 0.140 and 0.126 V, respectively. The potential shift would result from a lowering of activity of Np in Cd phase according to the alloy formation of NpCd
/Np couple at liquid Cd electrode at 723, 773 and 823 K were observed more positively than those at Mo electrode by 0.158, 0.140 and 0.126 V, respectively. The potential shift would result from a lowering of activity of Np in Cd phase according to the alloy formation of NpCd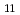 at 723 K and NpCd
at 723 K and NpCd at 773 and 823 K. The redox potentials of the Np
at 773 and 823 K. The redox potentials of the Np /Np couple at liquid Bi electrode at 723, 773 and 823 K were more positive than those at Mo electrode by 0.427, 0.419 and 0.410 V, respectively, which would be attributable to a lowering of activity of Np in Bi phase according to the formation of NpBi
/Np couple at liquid Bi electrode at 723, 773 and 823 K were more positive than those at Mo electrode by 0.427, 0.419 and 0.410 V, respectively, which would be attributable to a lowering of activity of Np in Bi phase according to the formation of NpBi .
.
Shirai, Osamu; Uozumi, Koichi*; Iwai, Takashi; Arai, Yasuo
Journal of Nuclear Science and Technology, 39(Suppl.3), p.745 - 748, 2002/11
no abstracts in English
Iizuka, Masatoshi*; Uozumi, Koichi*; Inoue, Tadashi*; Iwai, Takashi; Shirai, Osamu; Arai, Yasuo
Journal of Nuclear Materials, 299(1), p.32 - 42, 2001/10
Times Cited Count:76 Percentile:97.56(Materials Science, Multidisciplinary)no abstracts in English
 /Np couple in LiCl-KCl eutectic melts
/Np couple in LiCl-KCl eutectic meltsShirai, Osamu; Iizuka, Masatoshi*; Iwai, Takashi; Arai, Yasuo
Journal of Applied Electrochemistry, 31(9), p.1055 - 1060, 2001/09
Times Cited Count:21 Percentile:47.15(Electrochemistry)no abstracts in English
Shirai, Osamu; Iizuka, Masatoshi*; Iwai, Takashi; Suzuki, Yasufumi; Arai, Yasuo
Journal of Nuclear Science and Technology, 37(8), p.676 - 681, 2000/08
no abstracts in English
Shirai, Osamu; Iwai, Takashi; Shiozawa, Kenichi; Suzuki, Yasufumi; Sakamura, Y.*; Inoue, Tadashi*
Journal of Nuclear Materials, 277(2-3), p.226 - 230, 2000/02
Times Cited Count:24 Percentile:80.82(Materials Science, Multidisciplinary)no abstracts in English
Iizuka, Masatoshi*; Uozumi, Koichi*; Inoue, Tadashi*; Iwai, Takashi; Shirai, Osamu; Arai, Yasuo
Proceedings of 6th International Exchange Meeting on Actinide and Fission Product Partitioning and Transmutation (CD-ROM), p.327 - 341, 2000/00
no abstracts in English
Sakamura, Y.*; Inoue, Tadashi*; Shirai, Osamu; Iwai, Takashi; Arai, Yasuo; Suzuki, Yasufumi
Proc. of the Int. Conf. on Future Nuclear Systems (GLOBAL'99)(CD-ROM), 8 Pages, 1999/00
no abstracts in English
Kobayashi, Fumiaki; Ogawa, Toru; Okamoto, Yoshihiro; Akabori, Mitsuo
Journal of Alloys and Compounds, 271-273, p.374 - 377, 1998/00
Times Cited Count:8 Percentile:52.63(Chemistry, Physical)no abstracts in English
 -50mol%LiCl
-50mol%LiCl*; *; *;
J.Chem.Soc.,Faraday Trans.,2, 81, p.319 - 326, 1985/00
no abstracts in English
; H.Shimotake*
New Mater.New Processes, 2, p.283 - 288, 1983/00
no abstracts in English
; *
Yoyuen, 23(2), p.117 - 134, 1980/00
no abstracts in English
; R.S.Williams*; J.S.Wong*; D.A.Shirley*
Journal of Chemical Physics, 71(11), p.4601 - 4610, 1979/00
Times Cited Count:22no abstracts in English
 and their mixtures
and their mixtures; *; Furukawa, Kazuo; *; *
J.Chem.Soc.,Faraday Trans.,I, 74(7), p.1861 - 1870, 1978/07
no abstracts in English
; *
Nihon Genshiryoku Gakkai-Shi, 6(3), p.158 - 164, 1964/00
no abstracts in English
;
Nihon Genshiryoku Gakkai-Shi, 6(9), p.500 - 504, 1964/00
no abstracts in English